Montana IPM Bulletin, Fall 2017

The Montana IPM Bulletin presents critical pest management and pesticide education articles for Montana homeowners, pesticide applicators, farmers and ranchers. These articles are designed to deliver timely updates from an unbiased perspective that are specific to Montana. This is a cooperative effort between Montana State University pesticide education and integrated pest management programs.
Contents
- Cercospora Leaf Spot on Sugarbeet
- Weed Management Lessons from a Warm and Dry Summer
- Increasing Funding for Pesticide Education Trainings across Montana
- Common Burdock
- Preventing Herbicide Injury to Non-Target Plants: What Can You do as an Herbicide Applicator
- Ask the Expert
- Pest Management Toolkit
- Meet Your Specialist: Toby Day
- Acknowledgments
PDF links can be viewed with Adobe Acrobat or Sumatra PDF.
Cercospora Leaf Spot on Sugarbeet
Jessica Rupp, Extension Potato, Sugarbeet, and Pulse Pathology

FIGURE 1. Early infection of sugarbeet with CLS. Lesions are small and round with a dark brown border. Photo: Jessica Rupp
Cercospora leaf spot (CLS), caused by the fungus, Cercospora beticola, is considered the most important foliar disease of sugarbeet in Montana. Yield losses can approach 40% or more under conducive environmental conditions. Losses are attributed to loss of leaf area to the lesions, and the toxins, cercosporin and beticolin produced by the fungus. As the sugarbeet invests more energy to produce more leaves, sugars are unable to be stored in the root.
CLS produces round lesions that are very small, 3-5 mm in diameter. Spot centers appear light brown to brown (Figure 1). As the disease progresses the lesions may coalesce (Figure 2). The toxins cause rapid leaf death. Spots can also be found on petioles, where they may appear more elongated. Full leaf death of those leaves supplying most of the energy for sugar production can occur in serious infections (Figure 3).
The fungus survives between seasons in infected leaves and stems and requires water films, or very high humidity and temperatures ranging from 68°F to 79°F. The disease is most damaging in warm, humid summers. A full disease cycle can take place in as few as 10 days.
Weather models exist to help growers predict the environmental parameters for action, as well as yield loss components. Daily potential infection values (DIVs) are based on the number of hours per day where humidity is greater than 90% and hourly recorded temperature. These values are then expressed as a number between 0-7 using a model developed by Shane and Teng, 1984. These representative values are then added together for two-day totals to create a DIV of 0-14. Values of 7 or above indicate high risk. In Montana, values between 4-6 are considered conducive for disease development. It is critical to apply a fungicide when conditions first favor disease. Late application of the first spray often leads to difficult season-long control regardless of subsequent fungicide application timings.
When discussing fungicide resistance, it is important to understand that this designation means that the fungus is unaffected by the fungicide that previously gained control in the field. This differs from the term tolerance. In this instance, tolerance means that the fungus growth is reduced under a level of fungicide that previously prevented fungal growth. If tolerant strains are found in the field, growers can expect a reduced level of control. If resistant strains are present in the field, growers will see no control. Resistant isolates of CLS are present in states surrounding Montana, but only tolerant isolates have been found in Montana. It is very critical to rotate fungicide mode of action, both in season, and into the following season. If you suspect either scenario of tolerance or resistance in your field, please contact your local MSU Extension office or Schutter Diagnostic Lab, phone (406) 994-5150.

FIGURE 2. Cercospora lesions coalesce if the disease is allowed to progress. Photo: Jessica Rupp
It is recommended that growers be especially aware of CLS resistance to the benzimidazole class of fungicides; although not found yet in Montana. Because the potential development of fungicide resistance in this class is particularly high in Montana, MSU recommends that a tank mix be used with a benzimidazole (thiophanate methyl) and TPTH (triphenyltin hydroxide). Mix according to label instructions. Labels may be found on the Crop Data Management Systems website. Research from NDSU indicates that this fungicide combination works best when used as the first foliar fungicide application. This application is to prevent the disease and apply prior to identifying CLS in the crop. DIVs are reported by the Sugar Co-op agriculturalist in the region and should be used as the indicator of disease risk. Agricultural staff will provide advice on timing of the first spray application when environmental conditions are right for the disease. Never apply the same fungicide(s) or fungicide classes consecutively. Use at least ¾ rate of all fungicides applied in a tank mix.

FIGURE 3. Older leaves die due to Cercospora infection, thereby vastly reducing the plant's ability to store energy in the form of sugars in the root. Photo: Howard F. Schwartz, Colorado State University, bugwood.org
Instances of CLS isolates tolerant to TPTH have been around since the early 1990s in eastern Montana sugarbeet growing areas. This can be very challenging to identify at the field-scale as it can be difficult to tell if the cause is fungicide application problems or tolerant isolates. Lastly, resistant CLS isolates have been reported in North Dakota to the QoI fungicides, the strobilurins in 2016. Producers using products containing active ingredients in this family, especially pyraclostrobin, should be vigilant about rotation of mode-of-actions and field scouting.
QoIs, benzimidozoles, triazoles, ethylenebisdithiocarbamates (EBDC), and TPTH products are registered for use for controlling CLS. Consult product label for rates and pre-harvest intervals. Tank mixing of fungicides has proven to be a valuable approach to management.
Cultural actions can also be taken to address Cercospora leaf spot (CLS). Destroy weeds and any leftover debris prior to planting. Crop rotations of at least three years allow for the decay of any debris left in the field. Cultivation can speed debris degradation. When possible, use varieties with genetic resistance. Distances of at least 100 meters are recommended between current and previous fields. Do not plant into fields used for sugarbeets the previous season. Scouting should begin prior to the onset of row closure and continue throughout the season. If growers have had Cercospora leaf spot issues in previous years, they should consider planting a more tolerant variety.
For more information on fungicides or sugarbeet, please see CDMS, the North Dakota State University (NDSU) fungicide guide or the NDSU sugarbeet production guide on their publications page.
Weed Management Lessons from a Warm and Dry Summer
Fabian Menalled, Extension Cropland Weed Specialist
This summer we experienced very warm and dry growing conditions, and the heat and lack of rain severely damaged many crops. Many winter wheat growers were forced to cut their crop for hay because of low yield and quality. Thousands of spring wheat, pea and lentil acres were severely injured by the intense heat and lack of moisture. Conditions were such that in late June, Montana Governor Steve Bullock issued an executive order declaring a drought emergency in 19 northcentral and eastern counties and two American Indian reservations (Executive order at FMCSA; scroll to “Montana” and select “Executive Order 5-2017”).
Crops were severely damaged by the lack of rain, while farmers were confronted by increased weed management challenges. Low soil moisture resulted in many cases of increased weed competition and reduced control efficacy. The lack of control could cause problems in future years through the weed seeds that were produced.
Problematic weed species such as Russian thistle, common mallow, and kochia are drought tolerant, and their management became more difficult under low moisture conditions. Species with extensive and deep root systems, such as Canada thistle, were able to “mine” water from deep soil horizons. Winter annual species such as cheatgrass benefited from last year’s autumn rainfalls, which gave them a competitive advantage this summer.
Delayed weed emergence decreased the efficacy of early season management tactics sporadically across Montana. Also, late season herbicide applications were impacted by the dry and hot conditions. Selecting a proper herbicide application in warmer and drier environments is complicated as weeds usually produce thicker leaf cuticles, resulting in less herbicide absorption into the plant. Also, slow plant growth results in less translocation of the herbicide.
Soil applied herbicides are usually less effective in dry conditions as moisture is required for herbicide activation and uptake. This is especially true for pre-emergent herbicides which require rainfall or irrigation to move into the soil depth where weed seed germination occurs. These herbicides usually need rain or irrigation within two to three weeks of application to be effective, but in many cases optimal performance occurs when moisture is received within a day or two of application. Herbicides that remain on a dry soil surface may be blown away with soil particles, increasing the risk of herbicide injury if sensitive crops are planted close by. Finally, the slow herbicide degradation that occurs in dry conditions may result in future crop damage. To avoid this risk, farmers should keep good records and carefully read the label before planting sensitive crops such as peas and lentils.
Postemergence herbicides may represent a management option in dry conditions, but their performance is also challenged by hot temperatures and lack of moisture. As with soil applied herbicides, a post-emergence application needs to be absorbed and translocated to be effective. Under drought conditions, stressed plants tend to get dustier in dry weather conditions, reducing herbicide contact. Other weeds species, including common lambsquarters, develop thicker cuticles when stressed which may result in reduced herbicide absorption and uptake. Furthermore, drought-stressed plants slow their growth and do not efficiently move the herbicide within them. Finally, in many cases postemergence treatments cannot always be applied at the proper time due to uneven crop stands and multiple weed fushes that occur with dry conditions.
The efficacy of postemergence herbicide applications can be enhanced through proper selection of spray additives that improve herbicide coverage and absorption. Farmers should consult the herbicide label to see if the use of spray additives is allowed. For example, surfactants can enhance herbicide uptake by lowering evaporation of herbicide droplets, causing greater herbicide droplet contact with the leaf surface, and aiding in herbicide movement into stomata, but care should be taken as improper applications could synergistically enhance the detrimental impact of dry conditions.
Eventually it will start raining again. Hopefully, this fall, next spring and the following summer will bring much-needed moisture. Still, it will be wise to remember lessons learned this summer.
Increasing Funding for Pesticide Education Trainings across Montana
Cecil Tharp, Pesticide Education Specialist
Federal and state funding cuts have compromised many pesticide education programs across the nation. Some universities have closed the doors on pesticide education, while others offer fewer pesticide applicator training. Historically the Montana State University (MSU) Pesticide Education Program (PEP) coordinates certification and training of private applicators, which ensures applicators receive valuable education on the care and handling of pesticide products (Figure 4). In 2014 Crop Life Foundation recognized MSU PEP as funding deficient in a competitive national grant process. Grant funds were used to form an ideal educational vision for land-grant university pesticide education programs and form a strategy for obtaining additional sources of funding.

FIGURE 4. Photo: Cecil Tharp
Private Applicator Surveys
Two surveys sent to 4,500 Montana private applicators from 2014 - 2016 assessed the perspective of Montana applicators related to the: 1) importance of MSU PEP, 2) ideal role of MSU PEP, and 3) ideal funding options that minimize negative impacts. Ninety-two percent of private applicators considered the MSU PEP to be valuable to very valuable (n=656). Twenty-six percent of overall respondents indicated they would lose their private applicator license as a result of less training tools / props for trainers, less training for trainers to provide technical updates, and fewer fumigant education programs if funding wasn't found. 82 percent of private applicators surveyed indicated that a loss of their license would cause significant economic losses when facing a pest outbreak (a minimum of $1,000-$5,000 per outbreak / individual). 55% of private applicators indicated they would lose at least $5,000 dollars from one pest outbreak, while 33% indicated they would lose over $10,000 from one pest outbreak without their private applicator pesticide license. The need to seek additional funding for the MSU PEP was well-supported by pesticide applicators.
Montana Pesticide Education Stakeholder Team
A Montana Pesticide Education stakeholder team was formed to assess the needs of Montana pesticide applicators and associated stakeholders. This included Cecil Tharp (MSU Extension Pesticide Education Program), Krista Lee Evans (Montana Agribusiness Association), Tom Butcher (Montana Grain Growers Association), Becky Kington (Montana Weed Control Association), Heather Rimel (Montana Seed Growers Association), Jay Bodner (Montana Stockgrowers Association), and Jess Bandel (Montana Farm Bureau Federation). This committee, with assistance from the Montana Department of Agriculture (MDA), was crucial to determining the ideal role of MSU PEP by reviewing applicator surveys and feedback from their own groups/associations. The strategy for seeking funding was based heavily on private applicator feedback:
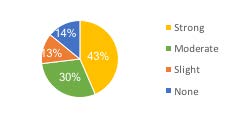
FIGURE 5. Applicator support for increased license fees: 43% strong, 30% moderate, 10% slight, and 11% none.
Private applicators (829) were asked if they would support an increase in private applicator license fees from $50 to $60. 73% of private applicators moderately or strongly supported this strategy. Only 14% indicated no support for this strategy (Figure 5). Consequently, this was a preferred avenue of support pursued (Figure 6).
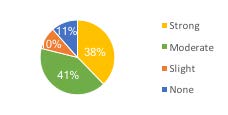
FIGURE 6. Applicator support for increased license fees: 38% strong, 41% moderate, 10% slight, 11% for none.
Private applicators (825) were also asked if they would support an increase in pesticide product registration fees to provide sustainability to the MSU PEP. Seventy-nine percent moderately or strongly supported this approach. Only 11% indicated they had no support for this strategy. Consequently, this was a preferred avenue of support pursued (Figure 6).
With applicator support the stakeholder team proposed an increase in private applicator license fees from $50 to $60 as well as raising pesticide registration fees to the MDA. These options were supported by MDA and later inserted into House Bill 126. House Bill 126 passed the Montana legislature in spring 2017.
Conclusion
The passage of House Bill 126 ensured adequate training for private applicators and the continued role of MSU PEP as the coordinators of the Montana Private Applicator Program. The increased funding will ensure applicators are trained to defectively manage pests while minimizing environmental or safety consequences from misuse.
An additional $10 in private applicator license fees will be dedicated towards MSU Extension county pesticide programs where the applicator resides. This funding can be used by local trainers to deliver high quality pesticide training programs. In addition, a portion of this funding is set aside for Tribal pesticide programs.
Private industry supported an additional $8 to $11 from pesticide registration fees to be collected from pesticide manufacturers wishing to register their pesticide products in Montana. This funding will be distributed to MSU PEP administration for: 1) training pesticide trainers to deliver local pesticide programs, 2) travel to support regional, state and local pesticide programs, 3) training tools for local trainers, and 4) increased regional initial and fumigant training opportunities.
This is good news for pesticide applicators in Montana. By maintaining a private applicator license with access to high quality pesticide programs, applicators save money by managing pests properly while ensuring the proper use of pesticides. Private applicators also will find it convenient to attend local training as opposed to traveling farther to attend statewide pesticide programs.
For more information on House Bill 126 navigate to LegiScan. Contact Cecil Tharp if you have any questions regarding this article (406-994-5067; [email protected]).
Common Burdock
By Jane Mangold, Extension Invasive Plant Specialist; and Stacy Davis, Research Associate
Common burdock (Arctium minus), also known as lesser burdock or wild rhubarb, is a tap-rooted forb in the Asteraceae family and native to Europe. After being accidentally introduced to North America in the 1600s, it has been reported in every U.S. state except Florida, Texas, and Hawaii and every Canadian province. Common burdock has been reported in at least 29 counties in Mon- Montana and is on 10 Montana county noxious weed lists (Big Horn, Blaine, Carter, Fallon, Hill, Lewis & Clark, Lincoln, Meagher, Pondera, and Stillwater); it is a noxious weed in Colorado, South Dakota, and Wyoming. Greater burdock (Arctium lappa) is a similar weedy burdock species that is less widespread in North America; it has been documented in at least 16 Montana counties.
First year common burdock plants form rosettes, while a stout, flowering stalk up to six feet tall forms the second year. Although commonly assumed to be a biennial, common burdock can behave as a perennial and take four or more years to flower under field conditions with moderate to high densities of non-weedy, desirable vegetation. If growing as a perennial, it usually dies after flowering (monocarpic). Large, heart shaped basal leaves resemble rhubarb (Figure 7) and are up to 1 ft. long with white and woolly undersides. They also have hollow petioles. Common burdock flowers from July to October. Flowers are pink to purple (rarely white) and are enclosed in a prickly bur (Figure 8). Flowering heads are typically less than 1.2 inches wide and are arranged in panicles with short stalks. Greater burdock differs from common burdock by its larger flower heads (greater than 1.2 inches) arranged in fat-topped clusters. In addition, the petioles on lower leaves are solid instead of hollow.
Common burdock can invade roadsides, stream banks, old fields, woodland edges, lawn edges, and waste areas while reproducing only by seed. While it thrives in areas of disturbance, it usually does not grow in areas that are severely disturbed on an annual basis, e.g., cultivated fields. One plant can produce 15,000 seeds. Flower bracts are tipped with hooked spines, which can easily attach to clothing or animal fur, thus dispersing the seed far distances. It has been suggested that seed viability for common burdock is only 1-3 years.

FIGURE 7. Large, heart-shaped leaves of common burdock. Photo by Matt Lavin, MSU.

FIGURE 8. Common burdock flowers are pink to purple and enclosed in a prickly bur. Photo by John Byrd, Mississippi State University, bugwood.org

FIGURE 9. Bird entrapped on common burdock burs. Photo by Matt Lavin, MSU.
Most impacts of common burdock are negative. The value of sheep’s wool can be reduced when burdock seed heads are entangled in it. Additionally, birds and bats can become trapped on the clusters of burs on the plant (Figure 9). Some Audubon chapters in Montana gather to pull common burdock to reduce impacts to birds. Common burdock is a secondary host for pathogens, such as powdery mildew and root rot, which can spread to economically important plants. On a positive note, some people use common burdock as a medicinal herb due to its anti-inflammatory, antioxidant, and anti-bacterial properties; and in Japan, burdock root is cultivated as “gobo” and is used in cooking.
Since common burdock is a prolific seed disperser, it is very important to limit seed production and prevent further spread. Hand-pulling or digging can be defective for small infestations; for example, the Sacajawea Audubon chapter in Bozeman, is eradicating common burdock in designated areas by repeated hand-pulling, as mentioned earlier. If digging, it is recommended to cut back the first year basal rosette and dig out the taproot entirely. Mowing is recommended after plants have bolted but before flowering. Buds can re-form after cutting, so monitoring afterward is essential. Cultivation is defective but is only feasible in certain areas. Herbicides are most defective when used at the rosette stage. Products containing the active ingredients 2,4-D, dicamba, or metsulfuron are defective. No biological control agents are available for common burdock. Livestock, primarily sheep, may eat burdock. In general, common burdock is not a difficult weed to control, but the task is easiest if infestations are managed when they are still small. Combining multiple control tactics, for example hand-pulling scattered plants in environmentally-sensitive areas, plus spraying with herbicides in areas where the weed grows more thickly, can be defective.
Preventing Herbicide Injury to Non-Target Plants: What Can You do as an Herbicide Applicator
By Noelle Orloff, Schutter Diagnostic Lab
Herbicides are an important tool for integrated weed management in croplands, rangelands, pastures, and in residential settings. However, this tool can sometimes have unintended consequences, causing injury to non-target plants such as crops, garden plants, trees, and other valuable vegetation. Herbicide applicators can prevent most of the common sources of non-target herbicide injury by reading and understanding the product label.
The Schutter Diagnostic Laboratory (SDL) at MSU has a front-row view into the issue of non-target herbicide injury. One of our roles at the SDL is assisting producers, pest management professionals, and homeowners with diagnosis of herbicide injury of plants. For our diagnoses we do not perform tissue tests to detect the presence of a given herbicide. Instead, we visually assess samples. This means we are never certain that an herbicide was involved, but we can match plant symptoms with herbicide modes of action. We have diagnosed 70 samples between May and September 2017 where herbicide injury was suspected as the main cause of plant symptoms. Leading sources of suspected injury have been herbicide drift, herbicide carryover in garden amendments, and herbicide carryover injury to pulse crops (i.e. peas and lentils).
Residential Herbicide Drift

FIGURE 10. Aspen showing symptoms of suspected herbicide drift. Photo: Noelle Orloff
Almost half of the herbicide injury cases submitted to SDL this year have been from suspected drift of plant growth regulator herbicides in ornamental settings. Plant symptoms include curling, twisting, stunting, and distortion that may be most pronounced on the newest growth (Figure 10). Plant growth regulators are a group of herbicides including many that are commonly used for broadleaf weed control in lawns such as 2,4-D and dicamba. In these cases homeowners or lawn care companies either applied these types of herbicides on the affected property, or we suspected drift occurred from another property. Some formulations of these herbicides can simply drift during application or can readily volatilize and drift relatively long distances in gaseous form during periods of high temperatures. Air temperature restrictions may be listed on the label to prevent issues of volatilization-based drift.
Herbicide Carryover in Garden Amendments
Some plant growth regulator herbicides are very persistent in the environment and are used to control broadleaf weeds in rangeland and pasture, including picloram or aminopyralid. These herbicides can persist in manure, compost, topsoil, and hay or straw for a number of

FIGURE 11. Tomato showing symptoms consistent with herbicide carryover. Photo: Noelle Orloff
years, and applying contaminated amendments to gardens or vegetable crops is akin to applying these herbicides to plants- they can cause symptoms of leaf distortion, stem cracking, and poor emergence (Figure 11). To reduce the prevalence of this issue, applicators should read and understand the product label. Labels on these herbicides are designed to prevent such contamination by specifying proper use of both the herbicides themselves and end-products such as hay. For example, aminopyralid products have supplemental labeling that includes information for producers wishing to distribute manure or hay offsite. This label includes a stewardship plan. See: Dow AgroSciences Supplemental Labeling (100KB PDF).
Herbicide Carryover in Pulse Crops
This year we have seen an increase in the number of pulse crop (i.e. chickpea, lentil, and dry pea) samples with symptoms of suspected herbicide carryover. Often the herbicides in question are in the ALS inhibitor mode of action group, and symptoms include malformation, stunting, poor emergence, and/or yellowing (Figure 12). Many of these cases are likely attributed to an application of a soil residual herbicide in past small grain rotations. Some of the products with potential issues

FIGURE 12. Chickpea showing symptoms of suspected herbicide carryover. Photo: Noelle Orloff
include those that have planting intervals for pulse crops of greater than 18 months, such as fucarbazone and sulfosulfuron. To reduce the prevalence of this applicators should carefully review the label for any applicable plant-back intervals, and be aware that weather conditions such as drought can decrease the rate of herbicide degradation. If a producer plans to add pulse crops into their rotation, they should check herbicide records to make sure herbicide residue will not damage their crop.
Herbicide labels contain vital information for reducing the risk of non- target herbicide injury. Restrictions and recommendations found on a product label may include those about weather conditions, re-crop intervals, and pre-harvest intervals, and understanding these will help avoid the types of non-target injury described in this article. Diagnoses at the SDL are based on visual assessment of plant symptoms only and are not proof that an herbicide was involved in a given case. If you would like to have plant tissue or soil tested for herbicide residue, contact the Analytical Lab on the Montana State University campus at (406) 994-3383. For more information see the MSU non-target plant toxicity home page, or if pursuing reimbursement for pesticide-related non-target damage, contact a Montana Department of Agriculture Enforcement Officer.
Ask the Expert
Q. Where do I find pesticide applicator programs in my area?
Cecil Tharp says: Pesticide applicator programs can be accessed in a variety of ways. If searching for private applicator initial training programs, navigate to the MSU Pesticide Education home page, then select “Private Applicator Program” and finally “Initial Private Applicator Trainings." Applicators may select the agenda of interest and view registration information.
On the drop-down box for “Category” select “60: Private Agricultural Plant Pest” or the desired commercial category. Finally, select the county of interest on the drop-down box for “County.” From the “Private Applicator Program” page, an applicator may also view MSU PEP sponsored regional events by selecting “Recertification Training Opportunities.” Scroll down to view all MSU PEP sponsored regional events.
Q. I’m interested in planting milkweed for monarch butterflies. Is it legal to plant milkweed in Montana?
Jane Mangold says: Yes, it is legal to plant milkweed in Montana. Milkweed is somewhat of a misnomer because it is not a “weed” in terms of a legally-designative noxious weed. In fact, there are seven species of milkweed (Asclepias spp.) that grow in Montana, and all are native to the state and region. In contrast, noxious weeds are not native to Montana, and it is not legal to intentionally grow them. Showy milkweed (A. speciosa) has the widest distribution across Montana. Note that milkweeds are toxic to livestock, with varying degrees of toxicity across species. In general, those milkweeds that have whorled leaves (verticillate) are more toxic than those with opposite leaves. If planting milkweed, you may wish to avoid areas that are regularly grazed by livestock.
Q: I saw a commercial saying that Roundup can be applied in lawns. Is that correct?
Fabian Menalled says: Partially correct. Roundup is the commercial name for glyphosate, a broad-spectrum systemic herbicide and crop desiccant. If you apply glyphosate to a lawn, you’ll kill it. Don’t do it!
However, a few months ago Monsanto, released Roundup For Lawns. The formulation of Roundup For Lawns is different than the one of Roundup as it does not include glyphosate, but a mix of different herbicides (MCPA, dimethylamine salt, quinclorac, dicamba, dimethylamine salt, and sulfentrazone) that have control on several broadleaf and grass weeds and can be applied to lawns containing the following species of grass: Kentucky bluegrass, perennial ryegrass, fescue species (including tall, red and fine leaf varieties), bermudagrass, bufalograss and zoysiagrass. This is a great example of why it is so important to carefully read the label before applying any product!
Pest Management Toolkit
South-Central Montana. October 2–6. 2017 Pest Management Tour. Fergus, Wheatland, Musselshell, Petroleum, Judith Basin, Golden Valley, Sweet Grass, Stillwater, Yellowstone, Big Horn, and Carbon counties.
The Private Applicator Training (PAT) region 5 (south-central MT) certification cycle deadline is December 31, 2017. Applicators must accumulate 6 credits prior to the January 1, 2018, deadline to maintain their private applicator license. Tree credits will be offered to audience members in the AM or PM session; or 6 credits for attending both sessions. See the complete agenda and follow registration instructions on the PAT website.
Montana Pesticide News Stories Website
Critical Montana pesticide updates and miscellaneous pesticide news stories can be viewed on the Pesticide News web page. Applicators may choose from four categories including:
County PAT Coordinator List
Applicators may look up their county PAT coordinator. This consists of MSU Extension Agents and Montana Weed District personnel. These contacts often can assist in delivering future pesticide program information before officially posted, answer private applicator certification questions and/or assist with pest recommendations.
Newly Updated Extension Bulletins
- Biology, Ecology and Management of Montana Knapweeds (2.4MB PDF)
- This publication provides information on spotted knapweed, diffuse knapweed, and Russian knapweed, a group of closely related noxious weeds that have invaded Montana. These weeds threaten long-term productivity of Montana grazing lands and wildlands. Successful management of knapweeds requires the use of integrated weed management strategies. Color, 19 pages.
- Biology, Ecology and Management of the Knotweed Complex (5.5MB PDF)
- Japanese knotweed, giant knotweed, Himalayan knotweed, and Bohemian knotweed are perennial plants resembling bamboo with their hollow stems and rapid, aggressive growth habits. The most common method of spread is rhizome fragments dispersed along waterways or in transported soil. Control options include repeated cutting or hand pulling and herbicides. Color, 18 pages.
- Biology, Ecology and Management of Hoary Alyssum (6MB PDF)
- Hoary alyssum is an exotic annual to short-lived perennial forb designated as a noxious weed in Montana since 2008. Maintaining healthy stands of vegetation and reseeding after major disturbances are the best ways to prevent establishment. Herbicides are an effective control option, and repeated applications may be necessary to treat plants that emerge throughout the growing season. Color, 14 pages.
Tools for Coping with Herbicide Damage
Losing a crop due to dry conditions or hail is a fatality that cannot be prevented. However, minimizing the risk of a pesticide application can be minimized. North Dakota State University has produced the following documents to help producers cope with pesticide damage:
- Documentation for Suspected Herbicide Spray Drift Damage, WC-751.
- The 2017 ND Weed Control Guide, includes a list of laboratories that conduct residue testing on page 106.
Also, the Cropland Weed Extension program has several resources including high resolution photos.
Meet Your Specialist: Toby Day, Extension Horticulturalist

Where/when did you receive your degrees?
I got both degrees at MSU, a BS in Horticulture/Landscape Design in 2000 and MS in Plant Sciences in 2006.
What is your field of interest (scholastic and research)?
Mostly consumer horticulture. I also have been working on tree fruit research for 4 years.
When did you arrive in Bozeman?
I arrived in Bozeman in 1973 at the Bozeman Deaconess Hospital on North Wilson. Ha!
Where are you from originally?
In my former life, I often like to think that I was a nobleman that was murdered by eating a meal poisoned by the Jerusalem cherry, as I don’t much like the taste of anything in the Solanaceae family. My wife likes to point out that I was likely the court jester/taster for the nobleman.
Where have you worked/taught in the past?
I started in Extension as an agent in Butte / Silver Bow County.
What are your hobbies?
Gardening, hunting, fishing, and rooting on the Pittsburgh Penguins are my four hobbies. I also like to ski, both downhill and cross-country.
What are some important areas of focus in your field?
Consumer Horticulture and tree fruit.
What are some of your current projects?
We have 10 research orchards across the state, as well as nearly 70 heritage orchards in Montana.
How can farmers use your research to their benefit?
Hopefully, they will be able to grow fruit as part of their farm.
What projects would you like to focus on in the future?
I would like to work on getting more publications and social media to clients across the state on consumer horticulture issues.
Acknowledgments
Do You Have a Question or Comment Regarding the Montana IPM Bulletin?
Send inquiries and suggestions to:
Cecil Tharp
Pesticide Education Specialist
P.O. Box 172900
Montana State University
Bozeman, MT 59717-00
Phone: (406) 994-5067
Fax: (406) 994-5589
Email: [email protected]
Web: pesticides.montana.edu
Jane Mangold
Invasive Plant Specialist
P.O. Box 173120
Montana State University
Bozeman, MT 59717-3120
Phone: (406) 994-5513
Fax: (406) 994-3933
Email: [email protected]
Web: landresources.montana.edu
Noelle Orloff
Associate Extension Specialist
P.O. Box 173120
Montana State University
Bozeman, MT 59717-3120
Phone: (406) 994-6297
Fax: (406) 994-3933
Email: [email protected]
Web: diagnostics.montana.edu

Common chemical and trade names are used in this publication for clarity by the reader. Inclusion of a common chemical or trade name does not imply endorsement of that particular product or brand of herbicide. Recommendations are not meant to replace those provided in the label. Consult the label prior to any application.
Original Fall 2017 PDF (2.6MB)
