Montana IPM Bulletin, Spring 2015

The Montana IPM Bulletin presents critical pest management and pesticide education articles for Montana homeowners, pesticide applicators, farmers and ranchers. These articles are designed to deliver timely updates from an unbiased perspective that are specific to Montana. This is a cooperative effort between Montana State University pesticide education and integrated pest management programs.
In this Issue
- Sustainable Management Strategies for Control of Flea Beetles
- Collecting and Submitting Plant Disease, Insect and Plant Identification Samples for Diagnosis to the Schutter Diagnostic Laboratory
- Bulbous Bluegrass (Poa bulbosa)
- Pest Management Toolkit
- Respiratory Protection of Increasing Concern for Montana Pesticide Applicators
- Meet Your Specialist: Emily Glunk
- Ask the Experts
- Acknowledgments
PDF links can be viewed with Adobe Acrobat or Sumatra PDF.
Sustainable Management Strategies for Control of Flea Beetles
Gadi V.P. Reddy, Superintendent & Associate Professor of Entomology/Insect Ecology, Western Triangle Agricultural Research Center, Conrad, MT

The crucifer flea beetle, Phyllotreta cruciferae (Goeze), has lately emerged as a serious pest of canola in Montana. The adult beetles feed on canola leaves, causing numerous small holes that stunt growth and reduce yield (Figure 1). In 2013, injury to canola seedlings was high (nearly 80%) in many parts of Montana. This demonstrates that when flea beetles emerge in large numbers they can quickly put an end to a young spring canola crop. Each year, yield losses due to flea beetle damage in the Northern Great Pains have been estimated to be tens of millions of dollars.
Flea beetles have a single generation per year and overwinter as adults in the leaf litter of shelterbelts or grassy areas; however beetles are rarely found in canola stubble. Beetles emerge when temperatures approach 57°F in early spring, proceed to feed on canola and weeds such as wild mustard and other brassicas and then move into newly sown canola as plants emerge. Depending on temperature, it may take up to three weeks for all adults to leave their overwintering sites. Adults feed on cotyledons and developing leaves and stems of seedlings, leading to loss of photosynthetic capability and finally plant death (Figure 2). Feeding starts within the first two weeks after beetle emergence and produces a shot-hole appearance and necrosis. Warm, dry, calm weather encourages flea beetle fight and feeding throughout the field, while at the same time slowing canola growth. In contrast, cool, rainy, and windy conditions decrease fight activity, but flea beetles walk or hop which leads to concentrations of beetles and injury along field margins.
The Western Triangle Agricultural Research Center (WTARC) near Conrad, Montana, carried out field trials during 2013 at two locations. The objectives were to determine nominal threshold levels for flea beetle on canola. The results indicated that a threshold of 15-20% leaf area injury is recommended for an insecticidal treatment in order to reduce the number of chemical applications and also reduce the possibility of selecting for resistance in flea beetles in Montana. However, in North Dakota, to protect the crop from yield losses, these insecticides are regularly applied at an economic threshold of 25% feeding injury to cotyledons and first true leaves.

Figure 1: Crucifer flea beetle, Phyllotreta cruciferae. Enlarged to show detail.

Figure 2: Damage to canola leaves caused by flea beetles.
Insecticide application is the main tactic for flea beetle management on canola, and the majority of canola acreages in North America are treated with insecticides. Classically, insecticide applications are made targeting adults in early spring when the canola crop is at the seedling stage, which is the most susceptible to flea beetle damage. In the Golden Triangle areas in Montana, canola growers traditionally use seed treatments. Neonicotinoid insecticides, applied either as seed treatment or foliar sprays are used as the main approach for the management of flea beetles. Insecticidal seed treatments with imidacloprid are used throughout the Northern Great Plains to systemically protect canola seedlings from flea beetle attack. Other approaches, including repeated applications of carbaryl, are often needed in order to keep flea beetles below economic injury levels; additionally, foliar spray of insecticides, i.e. deltamethrin or bifenthrin, are also used to prevent flea beetles from causing significant feeding injury. However, due to environmental concerns (i.e.impact on pollinators and natural enemies from the frequent and heavy use of chemical insecticides), more environmentally- compatible tactics are desirable for sustainable management of this pest.
The field experiments conducted by WTARC during 2013 indicated that combined use of entomopathogens (fungi), Beauveria bassiana (2.4 g/liter) and Metarhizium brunneum (5 g/liter) is more effective in reducing feeding injuries and improving yield levels when compared to chemical control and other treatment strategies. This indicates that entomopathogenic fungi are effective against flea beetles and may serve as alternatives to conventional insecticides or seed treatments in managing this pest.
Collecting and Submitting Plant Disease, Insect and Plant Identification Samples for Diagnosis to the Schutter Diagnostic Laboratory
Eva Grimme, Plant Pathology Diagnostician, Schutter Diagnostic Laboratory; Mary Burrows, Extension Plant Pathologist; and Laurie Kerzicnik, Insect Diagnostician
Plant diagnosticians at Montana State University in the Schutter Diagnostic Laboratory are available to identify any problems that affect plants in the landscape. Accurate identification of plant disorders and pests are the foundation for integrated pest management. A good quality sample and as much information as you can provide on the history of the problem is important for accurate, fast diagnosis and appropriate recommendations. To expedite identification and recommendations, please follow the following guidelines. Submission instructions, forms, and more information can be found on our website. You can drop samples of with your local county Extension agent or submit them to the lab directly. Your county agent is a good place to start – they are familiar with many of the plant disorders in the community, and may be able to identify your problem right away.
Plant Disease Specimens
-
Send sufficient plant material. Examine the entire plant for symptoms and collect samples that show various stages of the problem. Include a healthy plant or plant part for comparison. Send enough plant material so that an identification can be made. Whenever it is practical, include roots or the entire plant or clump of plants. If that is not possible, include a branch or send a twig. Detached leaves or parts of leaves are seldom useful.

Figure 3: Example of a well packaged sample (left) and an insufficient packaged sample (right)
- Avoid sending dead plants or plant parts since they are not useful for accurate diagnosis.
- Keep samples as fresh as possible until you can ship them. Avoid exposing the sample to direct sunlight and refrigerate if possible.
- Keep some soil around the root ball and of foliage. Wrap the plant sample in plastic and secure with a rubber band around the base of the plant (Figure 3). This prevents the soil from damaging the leaves during shipping. Loosely enclose the foliage in plastic or paper. Do not add water.
- Send a sample so that we could plant it when it arrived and it would survive.
- Package samples in crush-proof containers. Never send leaves in a fat paper envelope, the post office machinery causes extensive damage, and the tissue rots or dries out in shipping.
- Include photographs illustrating the problem if possible. Make sure photos are in focus, and include your name and contact information with sample submissions. Email the photographs to [email protected].
- Always include background information. Plant problems are often influenced by many different factors, so include as much information as possible: plant and variety, location (greenhouse, field, windbreak, home garden, etc.), irrigation practices (type of system, frequency, amount applied), history (age, size, amendments, fertilizer, etc.), pesticides used with names, rates, and dates, weather conditions, pattern of symptoms on the plant and surrounding plants, previous problems in this location. In-depth background information will help the diagnostician to identify the problem and is essential for timely management recommendations.
- Do not ship on Fridays. Samples can rot, bake or freeze over the weekend.
- Include a submission form, which can be found on our website or at your county Extension office.
If you have any questions about submitting a plant disease sample, please contact Eva Grimme, (406) 994-5150.
Insect Specimens

Figure 4: Example of how to submit a spider or insect sample.
If you are sure your problem is caused by an insect, please follow the protocol below. Collect several insect specimens if possible. Place specimens in sturdy clear containers with tight fitting lids that are strong enough to survive mail or courier services. Hard- bodied specimens can be sent either alive or in containers of rubbing alcohol. Aphids, mites, spiders, small flies and larvae need to be sent in rubbing alcohol (Figure 4). Caterpillars should be flash boiled before placing in rubbing alcohol. Turf insects can be sent alive in soil/root samples and mailed in a crush-proof box. For additional information please contact Laurie Kerzicnik, (406) 994-5704.
Describe damage of concern
If the insect is not included in the sample (damage sample only) describe the insect if possible (shape, body length, coloring, behavior).
Information to Include with Sample
You may wish to print out an Insect Identification Form to include with your sample.
Include the Following Collection Data
- Collection date.
- Collection location (e.g., Helena, MT). Or, give nearest town and distance/direction from it (for example, 7 mi. SW of Helena, MT).
- Collector’s name (not county agent etc.).
- Host identification (for example, green ash). If the host is not a plant, describe environment of collection site (for example, kitchen windowsill, basement, bag of rice).
Plant Identification
Plant diagnosticians in the Schutter laboratory can also help with plant identification. Please follow the instructions on how to collect a plant sample.
Short grasses, small flowering herbaceous plants, low shrubs: Collect several (at least three if possible) ENTIRE plants, including roots, plus extra flowers and/or fruits if available. Clean debris and soil from roots before shipping.
Tall grasses and shrubs, herbaceous forbs, trees, long vines: Collect several samples that adequately show stem features, leaves and leaf arrangement, and flowers and/or fruits. Collect extra flowers and/or fruits if available. If possible, collect or provide information about underground parts, ie. roots, rhizomes, bulbs, tubers, etc.
Record collection data and include state, county, geographic location of collection site, and date. This information is important to the identification process. Please remember to fill out a separate Plant Identification Form or Aquatic Plant ID form for each plant specimen/species submitted for identification.
Include a submission form.
If you have any questions about submitting a plant identification or aquatic plant ID sample, please contact the Schutter Diagnostic Lab.
Mailing Address for All Plant Samples
Schutter Diagnostic Lab
Montana State University
119 Plant BioScience Bldg
P.O. Box 173150
Bozeman, MT 59717-3150
Please mail a copy of the PDIS summary or other form with the sample.
Bulbous Bluegrass (Poa bulbosa)
Jane Mangold, MSU Extension Invasive Plant Specialist
Last growing season was a banner one for bulbous bluegrass (Poa bulbosa). Many people were not aware of this grass in spite of its presence in Montana since at least the 1930s. To better prepare for the 2015 growing season should it prove to be another banner season for bulbous bluegrass, this article provides a brief overview of the species, including identification, biology, impacts, and management recommendations.
Identification and Biology

Figure 5: Bulbous bluegrass plants. Photo by Matt Lavin, MSU.

Figure 6: Panicle with purple-based bulblets. Photo by Matt Lavin, MSU.

Figure 7: Stems with thickened, bulb-like bases. Photo by Jane Mangold, MSU.
Bulbous bluegrass is a relatively small, shallow- rooted, cool season perennial bunchgrass native to Eurasia and northern Africa (Figure 5). Because it grows new roots each season and has a relatively short life span, it is sometimes mistaken for an annual. However, it regenerates from its root system each year. The leaves of bulbous bluegrass are mostly basal and have a membranous ligule (thin membrane on the inside of the leaf blade at the junction of the sheath and blade). The lower stems are fattened, while the upper stems are wiry and round in cross-section. Bulbous bluegrass ranges in height from 8-24 inches. The panicle (inflorescence, or flowering and seed producing portion of the plant) is usually dense and has a plume-like appearance.
The most conspicuous feature of bulbous bluegrasss is the tiny bulblets with purple bases that form on the panicle (Figure 6). Bulblets provide the primary means of reproduction, and this reproduction is asexual. Bulblets can germinate immediately without a period of dormancy; in essence, each bulblet is a mini-plant that can form roots and grow once it falls to the soil surface. Bulblet viability is believed to be relatively short (two years). Bulblets can be moved via livestock, wildlife, infested hay, vehicles, and caching by small mammals. Bulbous bluegrass also has thickened and bulb-like stems at the base of the plant (Figure 7).
Impacts
Bulbous bluegrass was accidentally introduced to North America as a seed contaminant and was discovered in Oregon in the early 1900s. Because of its vigorous growth and tendency to form a solid mat, its use as a pasture and turf grass was repeatedly researched in the first half of the twentieth century; however, that line of research was abandoned after recognizing its invasive tendencies. Bulbous bluegrass is best adapted to disturbed, shallow soils that are moist during winter and early spring. It has been reported in nearly every state in the U.S., but is most common in the West.
Bulbous bluegrass produces very little biomass for grazing, and its palatability is confined to early to mid-spring before the grass dries out. The bulblets, however, contain high levels of starch and fat that are sought after by a variety of birds and small mammals. Bulbous bluegrass competes with more desirable vegetation in range sites and can also invade crop and hay fields.
Bulbous bluegrass is typically not competitive in dense stands of perennial crops like alfalfa or pasture. Therefore, maintaining desirable competitive vegetation can be very effective for preventing invasion by bulbous bluegrass or for reducing existing infestations. Disturbance promotes bulbous bluegrass; overgrazing and other disturbances that reduce vegetation vigor or create bare soil should be avoided.
Management
Hand pulling and digging can be used to manage bulbous bluegrass because of a shallow root system; however, it is difficult to remove all of the basal bulbs. Mowing is not considered to be effective and may even proliferate the problem by scattering bulblets. Spring tillage can reduce bulbous bluegrass and may be appropriate in some situations, especially if used in conjunction with seeding of other species during pasture renovation. Fall tillage is less effective.
Intensive early season grazing for several growing seasons can reduce bulbous bluegrass infestations. If grazing is going to be used, it must be applied early in the growing season because bulbous bluegrass generally grows earlier and faster than other perennial grasses. The effects of prescribed fire on bulbous bluegrass are not well known, but individual plants can be top-killed by fire. The survival of bulblets will depend on their location at the time of the fire and fire intensity. Bulblets are most likely to be killed by fire if they are on the plant. As they fall to the soil surface or become buried, they are more likely to survive prescribed fire.
Using herbicides that will selectively reduce bulbous bluegrass while not damaging desired grasses requires selecting the right herbicide and applying it at the correct time of the growing season. When using any herbicide, be sure to consult the label for more detailed information and always follow label directions. Several herbicides are labeled for bulbous bluegrass control in range and pasture. They include herbicides that contain the active ingredient glyphosate (e.g. Roundup), rimsulfuron (e.g. Matrix), sulfometuron methyl (e.g. Landmark XP), or sulfosulfuron (e.g. Outrider). Because glyphosate is non-selective, it should be applied early spring prior to active growth of desired perennial grasses. The other active ingredients listed above are more selective than glyphosate and can be applied post-emergent from fall to early spring. See table of herbicides from DiTomaso and Kyser (2013) for a list of herbicides that may be effective based on reports by researchers and land managers (PDF). When infestations are very dense and little desired vegetation remains, be sure to integrate revegetation with herbicide applications.
More information can be found on the MSU Extension Invasive Plants website.
Pest Management Toolkit
Initial Pesticide Applicator Training
April 24, Helena. This program can license individuals to apply restricted use pesticides on land they own, rent or lease. It is also worth 6 private recertification credits to licensed private applicators. For more information or to pre-register contact Brent Sarchet at (406) 447-8346. The agenda is viewable on the Pesticide Education Program website.
Montana Noxious Weed Awareness Week
June 8-12. To find events happening around the state during this week, go to mtweed.org or weedawareness.org.
2015 Montana State University Research Center Field Days
June 24 Northwestern, Creston; July 1 Northern, Havre; July 14 Eastern, Sidney; July 16 Central, Moccasin; July 17 Western Triangle, Conrad; July 21 Post Farm, Bozeman; July 23 Western, Corvallis; July 30, BART Hort Farm, Bozeman
Crop and Weed Field Day
July 21, Post Farm in Bozeman. The MSU Post Farm is 7 miles west of Bozeman.
Participants will be able to visit research and demonstration plots of weed management, pathogen control strategies, cropping systems, and crop traits. MSU Faculty, staff, and students will be available to answer questions. Attendees are eligible to receive Certified Crop Adviser (CCA) Continuing Education Unit credits as well as commercial and private applicator pesticide recertification credits. Please save the date and contact Fabian Menalled with questions.
Level 1 Noxious Weed Management Workshop
September 22-24, 2015, Bozeman. This is a 3-day study of weed biology, ecology, and management and is geared for weed management professionals, county Extension agents, and state and federal land managers. See the MSU Extension Invasive Plants website for more information.

Figure 8: Wheel of Weeds
Wheel of Weeds
Hosting a training or event and need a fun invasive plant activity? The Montana Noxious Weed Education Campaign has educational ‘Wheel of Weeds’ (Jeopardy-like) wheel game boards available for use (Figure 8). These ‘Wheel of Weeds’ games feature three different inserts: ‘Wildfower or Weed?’, ‘Integrated Weed Management’, and ‘Fight 5/Why YOU Should Care about Invasive Plants’. If interested in borrowing these fun educational items, contact Jane Mangold or Shantell Frame-Martin.
Play. Clean. Go (PCG)
PCG is a new nationwide weed education and outreach campaign geared toward outdoor recreationalists. The goal of PCG is to encourage people to take action to slow or stop the spread of invasive species while they partake in their favorite outdoor activities. For more information and to become a PCG partner, visit the Play Clean Go website.
Respiratory Protection of Increasing Concern for Montana Pesticide Applicators
Cecil Tharp, MSU Extension Pesticide Education Specialist
The uses of highly toxic pesticides that require respiratory protection were on the decline for over two decades. Recently, the use of one highly toxic active ingredient is on the rise due to glyphosate resistant kochia in Montana. This active ingredient is known as ‘paraquat’. Paraquat is in such formulations as Devour, Firestorm, Helmquat 3SL, Gramoxone SL, Cyclone SL 2.0, Bonedry, Willowood Paraquat 3SL, Paraquat Concentrate and Parazone 3SL Herbicide. Paraquat is a photosynthesis inhibitor and acts as a non-selective contact herbicide.
Applicators should use this pesticide product with care as it’s classified as a category 1 substance with the signal word of ‘Danger – Poison’. The signal word ‘Danger – Poison’ implies that this pesticide product is highly toxic through multiple routes of entry.
When using paraquat products applicators should remember to:
- Have buffers between the pesticide application and sensitive areas (livestock, people, pets).
- Follow all re-entry requirements on the pesticide products label.
- Wear proper personal protective equipment.
Personal Protective Equipment
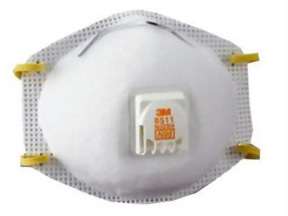
Respirator 1: Filtering face-piece respirator (N, R, or P)

Respirator 2: Air Purifying Respirator (APR) with particulate filters (N, R or P)

Respirator 3: Air Purifying Respirator (APR) with combination chemical cartridge and filter (N, R or P filter)
Personal protective equipment (PPE) requirements are usually on the first or second page of the product label under Precautionary Statements. List 1 represents the PPE requirements for applicators using the paraquat formulation known as ‘Paraquat Concentrate’. For applicators not mixing and loading, this includes protective eyewear, long sleeve shirt and pants, protective eyewear, chemically resistant gloves and the use of a NIOSH approved particulate filtering respirator with any N, R or P filter with an approval prefix of TC-84A (List 1).
List 1: Personal Protective Equipment Requirements for Paraquat Concentrate
- Applicators and other handlers (other than mixers and loaders) must wear:
- Long-sleeved shirt and long pants
- Chemical-resistant gloves - Category A (e.g., barrier laminate, butyl rubber, nitrile rubber, natural rubber, polyethylene, polyvinyl chloride (PVC or viton);
- Shoes plus socks
- Protective eyewear
- NIOSH-approved particulate filtering respirator equipped with N, R or P class filter media.
The respirator should have a NIOSH approval number prefix TC-84A. It is recommended that you require that respirator wearer be fit tested, and trained in the sue, maintenance and limitations of the respirator.
Respirator requirements can be quite confusing due to the wide variety of respirators on the market. Some product labels contain designations such as TC- 84A, TC-21A, TC-23C, TC-14G, TC-13F and TC-19C (List 2). The product label for ‘Paraquat Concentrate’ calls for a TC- 84A respirator.
The ‘Paraquat Concentrate’ product label further requires fit testing. Applicators using this product must wear any TC-84A option after proper fit testing. Applicators using any category 1 (Danger – Poison) liquid formulation are urged to wear only respirators that can be fit tested even if the product label doesn’t describe fit testing. There are many other types of respirators that may be required on other pesticide product labels including self-contained breathing apparatuses, powered air respirators and others (List 2).
List 2: NIOSH Designations
- TC-84A
- Filtering face-piece respirator (N, R or P)
- Air purifying respirator with particulate filers (N, R or P)
- Air purifying respirator with combination chem cartridge and filter (N, R or P)
- TC-21C
- Powered air-purifying respirator with particulate filter (HE)
- TC-23C
- Air purifying respirator with chemical cartridges
- Powered air purifying respirator with chemical cartridges
- Powered air purifying respirator with combination chemical cartridge and filter (HE)
- TC-14G
- Gas mask with or without particulate filter (N, R or P)
- Tight-fitting powered air purifying respirator with gas canister with or without filter (HE)
- TC-13F
- Self-contained breathing apparatus
- Supplied-air respirator with a self-contained escape bottle
- TC-19C
- Supplied-air respirator
Fit Testing
Fit testing is a method for selecting only respirators that fit properly. Pesticide applicators should perform a negative pressure test by covering the surface or hose where air is inhaled and breathe in. If the mask is properly sealed, it should collapse on face with no signs of leakage. Re-adjust mask until you get a seal or purchase a mask of a more appropriate size. Be aware that facial hair will obstruct the proper seal of a respirator. Even the face piece respirator has fit testing instructions that should accompany the respirator when purchased.
Types of Filters and Cartridges
The ‘Paraquat Concentrate’ product label also calls for the use of particulate filters rated as N (NOT resistant to oil), R (RESISTANT to oil) or P (oil PROOF). N-series filters are not oil resistant, R-series filters are oil-resistant and P-series filters are oil-proof. Filter efficiency is rated as 95, 99 or 100. For example you could have a label that specifies N, R or P filters with an efficiency rating of 100. This is referring to N100, R100 or P100 filters for your respirator. The product label for ‘Paraquat Concentrate’ allows for the use of any N, R or P filter.
Chemical cartridges remove gases and vapors but they don’t remove particulates. Organic vapor cartridges are the most common cartridges required for agricultural pesticides. Chemical cartridges should be changed if you detect chemical odors while wearing respirator.
For More Information
For more information on respirators see the CDC NIOSH requirements on Guide to the Selection and Use of Particulate Respirator or see pages 96 to 100 of the national pesticide applicator core manual [expired NASDA link]. For other questions contact Cecil Tharp, MSU Pesticide Education Coordinator.
Meet Your Specialist
Emily Glunk, MSU Extension Forage Specialist, Department of Animal and Range Sciences

I am originally from a small town in central Pennsylvania called Jersey Shore (and no, it is nowhere close to New Jersey). I got my undergraduate degree at Penn State University in 2010, then I went to North Carolina State University for my Master’s degree, which I completed in 2012. I then went directly into my doctoral degree at the University of Minnesota, which I just finished in 2014. I defended my Ph.D. the beginning of May, and headed west five days later. I got to Bozeman around May 15 of last year, and to my surprise, it was snowing. In May. Not something I expected. But I absolutely love Bozeman, and Montana, even with the weird weather we have been getting.
I was a teaching assistant at both NC State and University of Minnesota, and I also taught an Equine Forage Systems course at the University of Wisconsin-River Falls while I was working on my PhD. Teaching is one of my favorite parts of the job, and interacting with the students is one aspect I truly enjoy.
The things that I am most interested in are how to efficiently optimize forage production, whether it be at establishment, harvest, with rotations, fertilizer management, a number of things. I am also interested in the forage/animal interaction, as that is the reason that we produce most forages, to feed livestock. Looking at forage utilization and production in terms of animal production is really important, and collaborating with animal scientists in the department is something that I work hard to do. I have a lot of summer projects, including a new alfalfa variety trial, a forage establishment trial, a project looking at targeted grazing for termination of biennial clover, a nitrate quantification trial, and a forage-finishing project at Red Bluff.
I have tried to create a very applied research program that will be useful to producers and ranchers throughout the state. I have traveled quite a bit my first few months, talking to producers to find out what information they need for forage production systems.
As far as hobbies, I have two horses, my mare Khenya and her colt Django, which is where I spend the majority of my free time. I have had Khenya since I was in high school, and we competed in Dressage and hunter/jumpers. I’m hoping Django will be my next hunter prospect. I also enjoy hiking with my two dogs, Milly and Tucker, and doing anything outdoors.
Ask the Expert
Question: I read an article about a biopesticide for cheatgrass. Is there really something like this out there and if so, can you tell me more about it?
Jane Mangold says: Pseudomonas fuorescens is a naturally occurring, soil-dwelling bacterium that is currently being developed as a biopesticide for cheatgrass and some other weedy annual grasses. The isolate ACK55 selectively inhibits cheatgrass as well as medusahead and jointed goatgrass. Cheatgrass typically overwinters as a seedling and begins root growth in early spring before native range grasses and grassy crops like winter wheat resume growth. During this time, P. fluorescence ACK55 produces compounds that suppress root growth thus decreasing seedling vigor and the number of tillers and seeds produced. Over time (period of 3-4 years), the overall competitive ability of cheatgrass is reduced which allows neighboring desired grasses to out-compete cheatgrass. ACK55 and another Pseudomonas isolate, D7, are patented and in various stages of review and commercial development. D7 may be available as early as fall 2015; ACK55 would be available at some point after that, possibly fall 2016. A soil-borne fungal pathogen Pyrenophora semeniperda is also being explored as a cheatgrass biopesticide, but is not as far in its development as ACK55 and D7.
Question: Do I have any responsibilities to protect my workers from pesticides if I hire a commercial applicator?
Cecil Tharp says: Yes. You are still legally required to follow all federal Worker Protection Standard requirements even though you’re not applying the pesticide. As the owner of the area being sprayed you’re responsible for:
- Information at a central location (safety poster, restricted-entry interval [REI], application),
- Pesticide safety training
- Decontamination supplies
- Posted warning signs during REI
- Oral warnings
- Emergency assistance
Emergency assistance includes making transportation available in case anyone was injured by pesticides and making pesticide product labels readily available for emergency personnel. See complete WPS requirements on the EPA website. These requirements are mandatory if you have workers in the field up to 30 days after the spray application.
Question: Is there a good resource to identify weed seeds?
Fabian Menalled says: This is an excellent question as good weed management should consider all life stages of the species under consideration. Unfortunately, because it tends to be the least visible one, many times we forget to consider the seed stage. Yet, it is one of the most important stages in the life cycle of a plant, particularly of annual species, plants which grow from seed, reproduce, and die within a single year. Montana State University published the Weed Seedling Identification Guide, available as a spiral bound book on the MSU Extension store. The guide not only includes beautiful photos of weed seedlings and tips to recognize the species, but it also has photos of their seeds.
Acknowledgments
Do You Have a Question or Comment Regarding the Montana IPM Bulletin?
Send inquiries and suggestions to:
Cecil Tharp
Pesticide Education Specialist
P.O. Box 172900
Montana State University
Bozeman, MT 59717-00
Phone: (406) 994-5067
Fax: (406) 994-5589
Email: [email protected]
Web: pesticides.montana.edu
Jane Mangold
Invasive Plant Specialist
P.O. Box 173120
Montana State University
Bozeman, MT 59717-3120
Phone: (406) 994-5513
Fax: (406) 994-3933
Email: [email protected]
Web: landresources.montana.edu
Noelle Orloff
Associate Extension Specialist
P.O. Box 173120
Montana State University
Bozeman, MT 59717-3120
Phone: (406) 994-6297
Fax: (406) 994-3933
Email: [email protected]
Web: diagnostics.montana.edu

Common chemical and trade names are used in this publication for clarity by the reader. Inclusion of a common chemical or trade name does not imply endorsement of that particular product or brand of herbicide. Recommendations are not meant to replace those provided in the label. Consult the label prior to any application.
Original Spring 2015 PDF (3.8MB)
