Montana IPM Bulletin, Fall 2015

The Montana IPM Bulletin presents critical pest management and pesticide education articles for Montana homeowners, pesticide applicators, farmers and ranchers. These articles are designed to deliver timely updates from an unbiased perspective that are specific to Montana. This is a cooperative effort between Montana State University pesticide education and integrated pest management programs.
In this Issue
- Montana Noxious Weed List Revisions
- Biology, Ecology, and Management of Foxtail Barley
- Challenges of Managing Seed-born Fungal Diseases of Pulse Crops
- EPA Proposes New Pesticide Certification and Training Requirements for Licensed Pesticide Applicators
- Ask the Expert
- Pest Management Toolkit
- Meet Your Specialist: Noelle Orloff
- Acknowledgments
PDF links can be viewed with Adobe Acrobat or Sumatra PDF.
Montana Noxious Weed List Revisions
by Jane Mangold, Extension Invasive Plant Specialist

Figure 1. Phragmites (Phragmites australis ssp. australis) infestation. Photo by Bernd Blossey, Cornell University, Bugwood.org
The Montana noxious weed list was updated in July 2015. Tree new species were added and three species were moved to a different priority level. Recall that the noxious weed list categorizes species as Priority 1A, 1B, 2A, 2B, or 3. Weeds are placed into Priorities based on how abundant and widespread the species is across the state. Priority 1A weeds are not present or have a very limited presence in Montana; Priority 1B weeds have limited presence in Montana; Priority 2A weeds are common in isolated areas of Montana; and Priority 2B weeds are abundant in Montana and widespread in many counties. Priority 3 weeds are not noxious weeds, but regulated plants that have the potential to have significant negative economic and ecological impacts. Control of Priority 3 weeds is not mandated, but intentional spread or sale of them is prohibited.
Additions to List
Phragmites (Phragmites australis sub-species australis), also called common reed, was added as a Priority 1A noxious weed (Figure 1). Phragmites australis is widely distributed across the globe. Two lineages occur in Montana – one native (sub-species (ssp.) americanus) and one introduced from the Middle East and invasive (ssp. australis). Phragmites is an erect perennial grass that typically grows 6-15 feet high and has large (5-15 inches) feathery and plume-like inflorescences typically with a purple or golden hue. Stems are cane-like and up to 1 inch in diameter. Distinguishing between native and invasive Phragmites is difficult, and multiple traits should be considered. See Table 1 below for key features to examine between native and invasive Phragmites. In addition, native and introduced Phragmites habitats overlap extensively and include wetlands, along lakes, streams, and rivers, and near springs. Invasive Phragmites is more likely to occur in disturbed sites like roadsides and railways, construction sites, and near agricultural fields. Invasive Phragmites is very aggressive and can create dense monocultures that may alter wetland hydrology, increase fire potential, and degrade wildlife habitat of wetland-dwelling species.
Table 1: Helpful Features for Distinguishing Between Native and Invasive Phragmites
This table consists of 3 columns and 7 rows; column 1 and row 1 contain headings. Column 1 describes a common trait, then columns 2 and 3 note the differences in that trait when observing native and invasive Phragmites.
|
Feature
|
Native Phragmites
|
Invasive Phragmites
|
|---|---|---|
|
*Leaf sheath
|
Loose, fall away from stem; do not persist
|
Adhere tightly to stem; persistent
|
|
*Glumes (bracts at base of grass spikelet; usually 2, an upper and a lower)
|
Upper glume much longer (5-11 mm) Lower glume longer (>4 mm)
|
Upper glume shorter (4.5-7.5 mm) Lower glume short (<4 mm)
|
|
*Stem density
|
Less dense, typically mixed with other species; stems less persistent into next growing
season
|
Often grows in monoculture with high stem density; stems often persist into next growing
season
|
|
Spots on stems
|
May be present due to a native fungus
|
No spots, but maybe dark
smudges due to mildew |
|
Leaf color
|
Yellow-green to dark green
|
Dark green
|
|
Stem texture/color (observable when sheath is removed)
|
Smooth, shiny; reddish
|
Slightly ridged, not shiny; green
to tan |
*Most reliable features; adapted from Swearingen and Saltonstall (2010), National Park Service [expired link]
In August 2014 a population of the invasive Phragmites was confirmed in Hill County, MT. This is believed to be the first confirmed population in Montana, although this population has likely been present for some time. Additional surveys are being conducted to determine if existing Phragmites stands are native or exotic. Management is challenging and usually requires integrating mechanical, biological, and chemical methods. See the University of Nebraska-Lincoln Extension publication Noxious Weeds of Nebraska: Common Reed (1.3MB PDF) for more information.

Figure 2. Brazilian elodea (Egeria densa) foliage and flower. Photo by Leslie Mehrhoff, University of Connecticut, Bugwood.org

Figure 3. Parrot feather watermilfoil (Myriophyllum aquaticum). Photo by Leslie Mehrhoff, U of Connecticut, Bugwood.org
The two other species added to the Montana noxious weed list are Brazilian waterweed (Egeria densa) and parrot feather watermilfoil (Myriophyllum aquaticum), both of which were added as Priority 3 regulated plants. These species are aquatic herbs that are native to South America and were introduced to North America through the aquarium trade and as pond ornamentals. They can form dense monocultures in standing or slow flowing water bodies, especially those that are warm and have high nutrient availability. These thick monocultures can decrease water flow and clog irrigation equipment. Both species spread vegetatively by fragmenting. Fragments can be moved by boats and other aquatic recreational gear or by the careless dumping of aquarium plants. Once established, Brazilian waterweed and parrot feather watermilfoil are very difficult to control, so prevention of their establishment in Montana is critical.
Brazilian waterweed generally roots three to six feet below the water surface, but can root up to 20 feet below the surface. It is a bushy plant with dense whorls of bright green leaves. Each whorl has four to eight leaves and leaves are 0.5 to 1.5 inches long. The leaves are minutely serrated and linear. The undersides of the leaf midribs are smooth and have no teeth. Stems are erect, cylindrical, simple or branched, and grow until they reach the water surface where they form dense mats. Flowers ares mall (0.7 to 1.0 inch), extend about one to two inches above the water surface and have three white, glossy petals (Figure 2). Brazilian waterweed has not been reported in Montana.
Parrot feather watermilfoil gets its name from the feather-like, pinnately compound leaves arranged around the stem in whorls of five to six (Figure 3). Parrot feather has both submersed and emergent leaves. Submersed leaves are 0.5 to 1.5 inches long with 20 to 30 leaflets per leaf; emergent leaves are 1 to 2 inches long with fewer (6 to 18) leaflets per leaf. Emergent leaves are brighter green and stiffer than submersed leaves, and they can grow up to a foot above the water’s surface. Parrot feather has inconspicuous, white flowers about 1/16 inch long borne in the axils of emergent leaves. Male and female flowers occur on separate plants, and plants in North America are typically female. The first and only record of parrot feather in Montana was in 1977 near Stevensville (Ravalli County).
Changes in Priorities
The three species whose priority was changed include Eurasian watermilfoil, which was moved from Priority 2B to 2A; flowering rush was moved from 2B to 2A; and hoary alyssum was moved from 2A to 2B. Also see the updated Montana Department of Agriculture Montana Noxious Weed list.
Biology, Ecology, and Management of Foxtail Barley
Fabian Menalled, Extension Crop Weed Specialist

Figure 4. Foxtail barley (Hordeum jubatum). Photo by Steve Dewey, Utah State University. Bugwood.org
Farmers and ranchers across Montana are reporting increased trouble controlling foxtail barley (Hordeum jubatum) in no-till small grain, hay, and pasture fields. Understating foxtail barley biology and ecology will help producers develop long-lasting management strategies.
Because of its shallow and fibrous root, tillage has been an appropriate management option to control the spread of foxtail barley. Unfortunately, this species has become a problem in non-till and conservation tillage systems where soil disturbance is reduced.
Chemical management of foxtail barley in no-till cereal fields is difficult due to the reduced amount of effective in-crop herbicides and their high cost. Olympus (propoxy-carbazone) and Olympus Flex (propoxy-carbazone plus mesosulfuron-methyl) are viable options to control foxtail barley in wheat. Reports also suggest that Maverick (sulfosulfuron) can be used to control this weed in wheat. However, these product cannot be applied in barley, and it has very long residual activity.
Roundup (glyphosate) does kill foxtail barley seedlings, but repeated applications may be needed to control established plants. Previous research has shown that 0.98 lb/A of glyphosate applied in early fall can control up to 80% of foxtail barley plants. Adding ammonium sulfate (AMS) to glyphosate can significantly increase late-season control. Unfortunately, control drops to 50% when applied in late June, and almost no control can be expected during July and August.
In pastures, there are also few herbicide-based options to control foxtail barley. Plateau (imazapic) applied at 8 to 12 oz/A and Oust Extra (sulfometuron) at 2.6 to 3 oz/A can be used to manage this species, but both products need to be applied between late May to early June when the plant is rapidly growing but not yet setting seed. To improve control, it is possible to split the Plateau application into two 6oz/A applications. While this approach improves control, either application should be in early spring. Oust, a broad spectrum herbicide, can be used to control many broadleaf weeds in pastures, but it may reduce desirable grasses during dry years.
To overcome the limitations of an herbicide-based approach to manage foxtail barley, producers need to develop integrated management approaches tailored to the specific conditions of their systems. Producers should consider whether the ground is saline, seasonally wet, or overgrazed since these factors lead to infestations of foxtail barley. If this is the case, efficient irrigation and grazing management should be employed to keep the preferred species strongly competitive. Producers should be aware of a 12 to 48 month minimum plant back interval if converting pasture or rangeland to a crop.
In non-till and conservation tillage systems, farmers should ponder the potential use of cultivation to destroy foxtail barley root systems. As with many other weedy species, research has shown that cultural practices that promote the competitive crops help reduce yield losses due to foxtail barley. For example, increasing seeding rate significantly reduced foxtail barley biomass and seed production. However, narrowing wheat planting rows from 12 to 8 inches had little effect on foxtail barley abundance. Banding instead of broadcasting fertilizer is another approach to manage foxtail barley, because it gives crops a competitive advantage over the weed.
Although foxtail barley is not a highly competitive species, management choices or unfavorable environmental conditions can trigger its spread, particularly in reduced tillage systems. Establishing and maintaining healthy and vigorous crop and pasture stands help managing this species. In this context, preventing seed production and dispersal is key.
Challenges of Managing Seed-born Fungal Diseases of Pulse Crops
by Bright Agindotan, Research Assistant Professor, Regional Pulse Crop Diagnostic Laboratory, MSU-Bozeman
Pulse crops (edible seeds in legume family) including chickpea, lentil, and field (dry) pea are grown globally for their health and nutritional values. Their global acceptance is expected to increase as the world becomes more aware of their benefits and celebrates the “International Year of the Pulses” in 2016.
Montana is the largest producer and exporter of field peas and lentils in the United States. It produces 48% and 39% of U.S. field peas and lentils, respectively, in 2014. In the same year, 701,780 acres were planted, a 14% increase over 2013. Areas planted to field pea and lentil in 2015 also increased compared to 2014 with 570,000 acres planted to field pea (up 8%) and 180,000 acres planted to lentils (up 28%). About 70% of pulse crop seeds are shipped overseas.
As the acreage of any crop increases, so does the risk of infection by pests. Growers in Montana are aware of the fungal disease Ascochyta/Mycospharella blight and have been testing their seed for many years. This has benefited growers by eliminating heavily infected seed lots from the planted acres and reducing disease inoculum. However, there are other seedborne fungal pathogens that could limit pulse production in the future. Below is a list of seedborne fungi and their associated challenges.
Field Pea Ascochyta/Mycospharella
Field pea Ascochyta/Mycospharella blight is caused by three fungi: Ascochyta pisi, Mycosphaerella pinodes, and Phoma pinodella; however, they are caused by Ascochyta rabiei and Ascochyta lentis in chickpea and lentil, respectively. Varieties vary in their resistance, with kabuli chickpeas being the most sensitive to damage due to this disease. Complete defoliation and yield loss can occur if this disease is not managed. Field peas and lentils tend to be more tolerant to Ascochyta/Mycosphaerella blight, but crop rotation, choosing the best variety, using clean seed, applying seed treatments, scouting and fungicide applications are very important for management. Rotate fungicides to prevent the development of resistance. This is particularly important in chickpea, where resistance to fungicides including strobilurins or QoIs [Headline (pyraclostrobin), Quadris (azoxystrobin)] is common and resistance to succinate dehrogenase inhibitors (SDHI) [Priaxor (xemium), Endura (boscalid)] has been identified.
Botrytis (gray mold)
Botrytis is a seedborne pathogen (Figure 5). It is caused by Botrytis cinerea in chickpea, field pea, and lentil and can also infect a number of weed species. Its survival structure, called sclerotia, can remain viable in infected plant debris and soil for five years, providing a source of inoculum for future infection. The disease causes flowers to drop, resulting in significant seed yield losses. Seedling soft-rot can arise from infected seeds. There are no varieties resistant to this disease. Best management practices include testing seed, using a seed treatment [Stamina (pyraclostobin)], and applying a foliar fungicide [Headline (pyraclostrobin or Endura (bosaclid)], if needed.
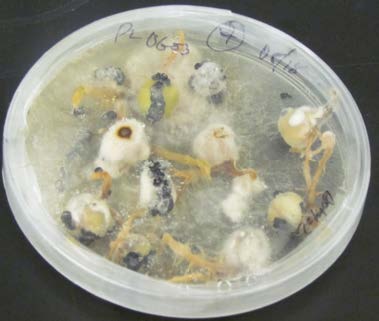
Figure 5a: Top view.
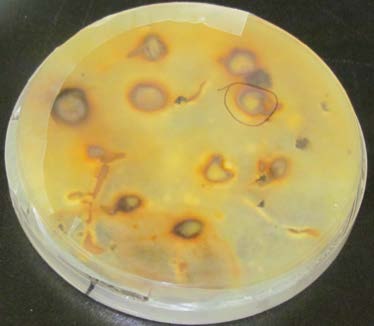
Figure 5b: Bottom view.
Botrytis (gray mold) infection of field pea seeds. The seed sample tested 0% for Ascochyta but 8% for Botrytis.
Alternaria Blight
Alternaria blight is caused by Alternaria alternata. At the Regional Pulse Crop Diagnostic Laboratory, we have isolated Alternaria from nearly all seed lots submitted for testing in fall 2015. The high infection rate and occurrence of the fungus tends to suggest that not all the isolates of Alternaria are pathogenic, or if they are, their transmission rate from seed to seedling is low. There is the need to characterize the Alternaria isolates from pea, lentil, and chickpea to determine their identity, pathogenicity, and seed transmission. It is unknown at this time how much of a threat this fungus is to seed quality or if it will cause significant disease in the crop.
Fusarium Wilt
Fusarium wilt is caused by Fusarium oxysporium. Each subspecies of the fungus infects specific crops. F. oxysporum F. sp. pisi infects pea, F. oxysporum F. sp. lentis infects lentil, and F. oxysporum F. sp. ciceris infects chickpea. There are several different races of the pathogen within each subspecies, which makes breeding for resistance challenging. We recently received funding from the Montana Department of Agriculture Specialty Crop Block Grant to conduct a survey to learn what subspecies of pathogen are present in pulse crops in Montana during 2015. At this time, crop rotation is the best management option. Fusarium wilt tends to show up at flowering.
Sclerotina (white mold)
Sclerotina is caused by Sclerotinia sclerotiorum, S. trifoliorum, or S. minor. The pathogens infect a broad host range, including pulse crops, mustards such as canola, and beans. They survive as a mass of fungal hyphae enclosed in protective cases known as sclerotia. The fungi will kill tissue and fill the stem with white hyphae and sclerotia, which then survive in the soil for many years. The disease lowers seed quality, as well. Management is extremely difficult and involves a multi-pronged strategy including crop rotation, tillage where available, and a limited number of fungicide options [Endura (bosaclid)] that must be applied at an optimal timing.
Stemphylium Blight (chickpea, lentil)
Stemphylium blight is caused by Stemphylium botryosum. It occurs in Montana and North Dakota. The fungus also infects field pea. It causes leaf spots which coalesce causing defoliation of the plant. The pathogen is seedborne and infected seeds that have a low germination rate. There are no fungicides registered for control of this disease. It often comes in late in the growing season and can cause seed staining.
Because pulse crop growers in Montana have not been testing for seedborne fungi apart from Ascochyta, there have been many cases where seed samples have 0% Ascochyta-Mycospharella spp. but contained high levels of other seedborne fungi: 5-8% Botrytis spp. or 10-34% Alternaria spp., or a mixture of these and other fungi. To address these issues and others, the new Regional Pulse Crop Diagnostic Laboratory in Bozeman, Montana, has started offering a Fungal Scan to identify all seedborne fungi of pulse crop seed. The lab intends to characterize fungal isolates we collect and use this information for educational outreach. For more information about seed testing services, visit the Regional Pulse Crop Diagnostic Laboratory website.
Disease identification resources can be found at your local county Extension office, the Schutter Diagnostic Laboratory, and the Diseases of Cool Season Legume (PDF). Accurate identification is very important for disease management.
Seed testing of all seedborne fungi of pulse crops is a smart and cost-effective way to manage the diseases. Healthy seeds, healthy start!
EPA Proposes New Pesticide Certification and Training Requirements for Licensed Pesticide Applicators
by Cecil Tharp, Montana State University Pesticide Education Specialist
Pesticide applicators should be aware of an EPA issued comment period that began on August 24th, 2015, that revises the current Certification of Pesticide Application rule. The EPA is accepting comments on this proposal during a 90 day comment period that ends November 23rd, 2015. This is expected to prevent up to 800 acute illnesses per year while better protecting the environment. Montana commercial, private, and non-certified applicators will be impacted in a variety of ways. Readers should review the following changes and some items that will remain similar. This is your opportunity to persuade EPA to alter potential rule changes and suggest better alternatives.
Complete details of the proposed revisions and directions for commenting are available on regulations.gov, under docket # EPA-HQ-OPP-2011-0183. After the 90 day public comment period, EPA will review and consider the public comments, and where needed, change the proposed requirements. EPA will draft the final regulation which will be reviewed by the U.S. Department of Agriculture and the Office of Management and Budget. When these reviews are complete, the EPA will issue the final regulation.
Current and Proposed Rule Tables
The tables below represent only some of the changes proposed by EPA. More details are available by accessing the EPA comparison on the MSU Pesticide News webpage. Each table contains two columns; column 1 contains the proposed rules, and in column 2 are the current rules.
Private Applicators
|
Proposed Rules
|
Current Rules
|
|---|---|
|
Closed Book Testing
|
Open Book Testing
|
|
Option to attend initial program (ungraded)
|
Option to attend initial program
|
|
Category Specific Training (i.e. Fumigation, Ag Pest)
|
No Category Specific Training
|
|
3 year certification cycle
|
5 year certification cycle
|
|
Minimum 18 years old
|
Minimum 16 years old
|
|
To renew must obtain 6 CEU’s in pesticide core
|
To renew must obtain 6 CEU’s
|
|
To renew must obtain 3 CEU’s in category specific
|
No category training required at all
|
|
Half credits must be accumulated in last 18 months of cycle
|
CEU’s accumulated any time in cycle
|
Non-Certified Applicators (under another individual's license)
|
Proposed Rules
|
Current Rules
|
|---|---|
|
Annual Safety Training
|
Must be competent to use RUPs
|
|
Certified applicator can provide training
|
Applicator provides instructions
|
|
Training must meet certain requirements
|
Requirements not stated; must ensure competence in using pesticides
|
|
Minimum 18 years old
|
Minimum 16 years old
|
Commercial and Government Applicators
|
Proposed Rules
|
Current Rules
|
|---|---|
|
Record keeping must be included in non-certified applicator training
|
No record keeping requirements for training non- certified applicators
|
|
Minimum 18 years old
|
Minimum 16 years old
|
|
3 year certification cycle
|
5 year certification cycle
|
Further Information
For details regarding the proposed action see the EPA website. For questions regarding implications in Montana, contact Cecil Tarp, Pesticide Education Specialist (406-994-5067; [email protected]). For technical questions or further details regarding commenting, contact Michelle Arling ([email protected]).
Ask the Expert
Extension IPM specialists provide answers to reader questions.
Question: I never had problems with green foxtail but after three years of planting spring wheat, my fields are infested with this weed. What can I do?
Fabian Menalled says: While herbicides can be used to control green foxtail (Setaria viridis) in spring wheat, it may be worthwhile to consider why it has become so dominant. There are two issues you should ponder. First, green foxtail populations have been reported in Montana to be resistant to Group 1 (ACC-ase inhibitors) herbicides such as Hoelon (diclofop-methyl), Fusilade® (fuazifop-P-butyl), Axial (pinoxaden), and Poast (sethoxydim). If you have used any of these products, you should check for the presence of herbicide resistant biotypes. Second, from an ecological point of view, green foxtail and spring wheat are so similar that every time you seed your crops you are opening the niche where green foxtail thrives. As a summer annual grass, green foxtail reproduces by seed with seedling emerging in early spring from shallow depths. Within six to eight weeks of germination, green foxtail seedlings can grow to maturity and produce seeds. Seeds usually remain viable up to three years, a relatively short time when compared with other weed species. Including winter wheat or pulse crops in your rotation are simple tools that will help you reduce green foxtail dominance and prevent the selection of herbicide resistance.
Question: I attended a pesticide education event for credits and later learned it was only awarded commercial pesticide applicator credits yet I have a private applicator license. Will commercial applicator credits transfer as private applicator credits?
Cecil Tharp says: No. Commercial applicator credits don’t equal private applicator credits. In Montana the reviewing and approving of pesticide applicator credits is conducted by separate agencies. Commercial applicator credits are reviewed by the Montana Department of Agriculture, while private applicator credits are reviewed by the MSU Extension service. It is the pesticide applicators responsibility to attend the properly accredited events. It is still possible to receive private applicator credits; however the program sponsor will need to forward the program agenda and sign in sheets to the MSU Extension Pesticide Education Program with the intent to submit for private applicator credits.

Question: We have a garden plot that is infested with field bindweed. There was nothing planted in this area this year. Would it be OK to spray it with glyphosate this fall and then plant potatoes or other vegetables next year?
Jane Mangold says: Yes, it would be OK to spray with glyphosate this fall and then plant next year because glyphosate doesn’t retain its herbicidal properties once in contact with the soil. Field bindweed is very difficult to eradicate once it is well established, so you may even need to apply glyphosate multiple times and combine it with some hand pulling for those plants that come back after the herbicide application. Be sure to apply the glyphosate on a warm, sunny day. It may take a week or two to start seeing its effects on your field bindweed.
Question: Why is everyone so concerned about Fusarium head blight in wheat?
Mary Burrows says: Fusarium head blight is a very serious disease of wheat and barley. It causes yield reduction and rejection of the grain due to a vomitoxin, deoxynivalenol, more commonly known as DON. Grain can be rejected for containing > 1ppm for human food and > 5 ppm for animal feed. There is a 0 ppm tolerance in malt barley because it causes beer to gush.
Fusarium head blight (scab) has occurred sporadically in some areas of the state such as the Yellowstone Valley, Gallatin Valley, and Fairfield bench for many years. It is increasing in prevalence largely due to increasing corn acreage, continuous cereal rotations, no-till, and the prevalence of susceptible varieties. Fungicides can be used, but are only suppressive and must be used in a very tight time window with the right nozzles and sufficient water.
More information can be found in the MontGuide Fusarium Head Blight (Scab) of Wheat and Barley
Pest Management Toolkit
New Montana State University Extension Publication
- Guide to Exotic Thistles of Montana and How to Differentiate from Native Thistles covers identification of 5 exotic and 10 native thistles found in Montana. Five exotic and ten native thistles grow in Montana. This publication is designed to determine whether an unknown thistle is exotic or native. If exotic, the publication will help you determine it to species. This publication also includes instructions on how to use a dichotomous key, descriptive text, and pictures to illustrate the exotic and native thistles that grow in Montana. 16 page booklet, color. Publication EB022.
Montana Weed Control Association Annual Conference
- January 12-14, 2016, at the Heritage Inn in Great Falls, MT.
- Visit mtweed.org for more information.
Do You Want to Learn More about the Climate and Agriculture Across the Northern Plains?
The USDA has Recently Established the Northern Plains Climate Regional Hub to provide "science-based knowledge, practical information, management/conservation strategies, and decision tools to farmers, ranchers, forest landowners that will help them to adapt to weather variability and changing climatic conditions." The Hub provides educational material including decision making tools as well as weather and climate tools. The information is arranged in four main topics: croplands, forestlands, rangeland and pastures, and livestock and can be found on the Climate Hub website.
Online Private Applicator Credit Opportunities, 2015
A variety of online programs are available for applicators desiring private applicator credits. Applicators may accrue up to 2 private credits every certification cycle. Instructions vary depending on the course you select.
For more information contact Cecil Tarp at (406) 994-5067. To view online programs navigate to the MSU Pesticide Education Program's Private Applicator Training webpage.
New Calibration Tool is available as a Mobile App; Spray Calc
University of Illinois Extension has released a new smartphone app for making sprayer-related calculations. Pesticide Spray Calculator, or Spray Calc, is available for both Apple and Android smartphone platforms. It contains multiple functions related to calibrating a sprayer. Navigate to the University of Illinois Pesticide Safety Education Program website for more information and links to download the app.
Meet Your Specialist
Noelle Orloff, Plant Identification Diagnostician and Associate Extension Specialist, Schutter Diagnostic Lab

Where/when did you receive your degrees?
I received my bachelor’s degree in Natural Resource Conservation from the University of Montana’s School of Forestry in 2003. In 2011, I received my master’s degree in Land Resources and Environmental Sciences at MSU where I focused on invasive plant ecology and management.
What are the responsibilities of your current position?
I work in the Schutter Diagnostic Lab, where we support Extension by helping homeowners, land managers, and producers with a wide range of plant issues. My main role in the lab is identifying plants. I also assess plants for herbicide injury and other abiotic problems in urban and agricultural settings. I started working in Schutter in May 2015.
When did you arrive in Bozeman?
I moved to Bozeman in April 2009, just in time to start field work for my master’s degree projects.
Where are you from originally?
I was born and raised in Rockford, Illinois.
Where have you worked/taught in the past?
I have worked quite a few places, so I will give you some highlights. The last six years I have spent working in Jane Mangold’s lab at MSU, and before that I spent several years working for the Rocky Mountain Front Weed Roundtable in Choteau, Montana. I have also worked at a few ski areas including Teton Pass in Choteau, Grand Targhee in Idaho, and Moonlight Basin here in Bozeman.
What do you like to do in your spare time? Any hobbies?
As you might be able to tell from my last answer, skiing is a hobby of mine. I am also an avid gardener and I love taking my elderly dog for walks.
Describe some of your past research projects.
I have worked on several projects in my past six years at MSU. I have done quite a bit of research about ecologically-based management of cheatgrass and other exotic annual grasses, including both field and greenhouse studies. I have also done research about how native plant communities respond to weed control with herbicides.
What are some of your current projects?
My main focus right now is developing Extension programming to train our agents, land managers, producers, and homeowners about plant identification. I am also starting to work on Extension materials to help with understanding herbicide carryover in soil amendments in home gardens, and identification of the plants newly added to the Montana noxious weed list. Finally, this winter I will wrap up a research project looking at management of perennial weeds, specifically Canada thistle and field bindweed, in organic agricultural systems.
How can farmers use your work to their benefit?
The first step in understanding any plant problem is knowing what plant you are dealing with! I help with that step of IPM by providing plant identification services, as well as Extension programming and publications that empower farmers to identify and better understand the species they are working with.
What projects would you like to focus on in the future?
I am excited to continue working with Extension agents and land managers to help people who work with plants better understand and feel more comfortable with plant identification.
Acknowledgments
Do You Have a Question or Comment Regarding the Montana IPM Bulletin?
Send inquiries and suggestions to:
Cecil Tharp
Pesticide Education Specialist
P.O. Box 172900
Montana State University
Bozeman, MT 59717-00
Phone: (406) 994-5067
Fax: (406) 994-5589
Email: [email protected]
Web: pesticides.montana.edu
Jane Mangold
Invasive Plant Specialist
P.O. Box 173120
Montana State University
Bozeman, MT 59717-3120
Phone: (406) 994-5513
Fax: (406) 994-3933
Email: [email protected]
Web: landresources.montana.edu
Noelle Orloff
Associate Extension Specialist
P.O. Box 173120
Montana State University
Bozeman, MT 59717-3120
Phone: (406) 994-6297
Fax: (406) 994-3933
Email: [email protected]
Web: diagnostics.montana.edu

Common chemical and trade names are used in this publication for clarity by the reader. Inclusion of a common chemical or trade name does not imply endorsement of that particular product or brand of herbicide. Recommendations are not meant to replace those provided in the label. Consult the label prior to any application.
Original Fall 2015 PDF (5.7MB)
